![]()
Chloroauric Acid
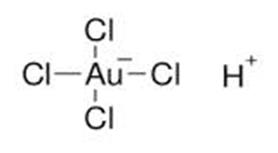
![]()
QH, Niloy Kumar Das
Shahjalal
Science & Technology University, Bangladesh
![]()

“I decided to
dissolve it.”
- Georgy de Hevesy
When Germany invaded Denmark in World War II, Hungarian chemist George de Hevesy dissolved the gold Nobel
Prizes of German physicists Max von Laue (1914) and James Franck (1925) in aqua regia to
prevent the German soldiers from confiscating them. Later the medals were
reconstructed from disolved chloroauric
acid and handed over to its rightful owners after the war.

Pic. György de Hevesy
Chloroauric acid is an inorganic acid that is widely used in gold
refining processes. Gold is one of the least reactive metals but gold reacts
with aqua regia to yield chloroauric acid.
Au + HNO3 + 4 HCl →
HAuCl4 + NO + 2 H2O
Chloroauric acid shows acidic behavior by releasing proton in
solution. It is a strong monoprotic conjugate acid.
Even if chloroauric acid forms in aqueous solution,
such solution are unstable due to hydrolysis of tetrachloriodoaurate ion.
H3O+ → H2O + H+
H3OAuCl4 + 2 H2O ![]() Au(OH)3 + 4 HCl
Au(OH)3 + 4 HCl
Gold is oxidized by halogens; so a solution of HAuCl4 can be obtained by the action of chlorine or chlorine water on metallic gold in hydrochloric
acid. Oxidation of gold by chlorine is used to recover gold from anode muds.
2Au + 3Cl2
→2 AuCl3
2
Au + 3 Cl2 + 2 HCl → 2 HAuCl4
There are
many reducing agents that reduce ionic gold into the metal, such as: SMB (sodium
metabisulfite), copperas (Iron II Sulfate), Sulfur dioxide
gas (SO2), hydroquinone, formaldehyde, hydrazine sulfate and many
more.
Major Applications
·
Gold refining:
Traditionally
chloroauric acid has been used to refine gold. Gold became
the basis of money in many ancient civilizations, and even today most countries
maintain large reserves of gold for financial credibility.

Front and
back of a coin from King Croesus’s mint – one of the first coins minted in
human history, over 2500 years ago.


American Gold Eagle; an official gold bullion coin of the United
States
·
Gold nanoparticles:
Today
with the development of nanotechnology; gold nanoparticles of 5 nm to 400 nm in
diameter have been produced from chloroauric acid. An
interesting fact is that the process requires traditional reducing agents in
one or another form. Some practical applications of gold nanoparticles are
listed below.
1.
Gold nanoparticles are designed for use as conductors
from printable inks to electronic chips.
2. Near-IR
absorbing gold nanoparticles (including gold nanoshells
and nanorods) produce heat when excited by light at
wavelengths from 700 to 800 nm. This enables these nanoparticles to eradicate
targeted tumors.
3. The large
surface area-to-volume ratio of gold nanoparticles enables their surface to be
coated with hundreds of molecules (including therapeutics, targeting agents,
and anti-fouling polymers).
4. Gold
nanoparticles are used in a variety of sensors. For example, a colorimetric
sensor based on gold nanoparticles can identify if foods are suitable for
consumption.
5. Gold
nanoparticles also scatter light and can produce an array of interesting colors
under dark-field microscopy. The scattered colours of
gold nanoparticles are currently used for biological imaging applications.
6. Gold
nanoparticles are also used to detect biomarkers in the diagnosis of heart
diseases, cancers, and infectious agents.
7. Gold
nanoparticles are used as catalysts in a number of chemical reactions. The
surface of a gold nanoparticle can be used for selective oxidation or in
certain cases the surface can reduce a reaction (nitrogen oxides). Gold
nanoparticles are being developed for fuel cell applications.
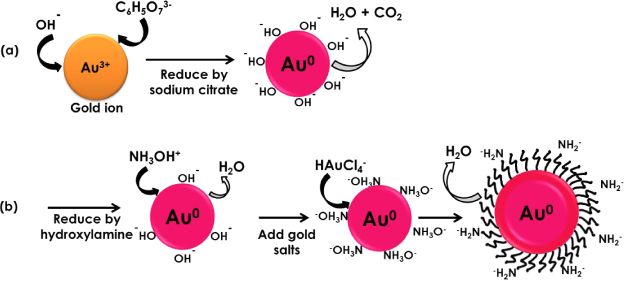
(a) Gold salts
are reduced by simple fatty acids to colloidal gold; (b) Additional functional
groups are attached to the gold nanoparticle by chemical treatment
·
Gold toner
One of the most popular uses for chloroauric acid is in photography as a gold toner. Toning is a chemical process which changes the colour of a photograph. It has a further benefit in that a toned image is far more permanent. In 1840 Frenchman Hippolyte Fizeau created a gold chloride toning bath to increase the stability of Daguerreo-type images. A black and white print toner that is commonly used in photography is actually created from real metallic gold. This process sees gold metal deposited onto the silver image. There are many different reasons for this. The main reason is that silver will eventually tarnish, however gold will not. As a result gold toning has proven to be one of the most effective processes for images, especially if printed on a well-washed archival fibe paper.
KAuCl4 +
Ag → Au + AgCl + KCl

Gold toned photo;
courtesy Andrew Sanderson
·
Glass coloring agent
Cranberry
glass or 'Gold Ruby' glass
is a red glass made by adding chloroaurates
or colloidal gold to molten glass.


Vintage
cranberry glass bowl, scent bottles
References
1. Feather, A; KC
Sole; Lj Bryson (July 1997). "Gold refining by solvent extraction—the minataur™ process" (PDF). Journal
of the Southern African Institute of Mining and Metallurgy: 169–173. Retrieved 2013-03-17.
2. Morris D. F. C.,
Khan M. A. "Application of solvent extraction to the refining of precious
metals, Part 3: purification of gold" Talanta, 1968. 15,
1301—1305.
3. Birgitta Lemmel (2006). "The Nobel Prize Medals and the Medal for the
Prize in Economics". The Nobel
Foundation.
4. T.G.H.
James, The British Museum, Gold Technology in Ancient Egypt: Mastery of Metal
Working Methods., 1972, Gold Bulletin V, p42.
5. Encyclopædia Britannica 1911, Alchemy
6. Chloroauric acid; ChemSpider
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15097203]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15097203]